The main component of SAP is sodium polyacrylate, which is made through polymerization reaction. Its molecular structure contains a large number of hydrophilic groups (such as carboxyl groups), which can quickly absorb and lock in moisture.
item no.:
Japan-branded SAPPayment:
TT, L/Cproduct origin:
JapanColor:
whiteshipping port:
XiamenLead Time:
15daysSAP Made in Japan for Sanitary NapkinSAP Made in Japan for Sanitary Napkin
Quick Details
Place of Origin:Japan
Another name: Sodium Polyacrylate/Super Absorbent Material
Brand: Sumitomo, San-dia, NSKK
Function: Absorbing and retaining liquids under pressure in sanitary napkin

SAP (Super Absorbent Polymer) used in sanitary napkins is a key material that is mainly used to quickly absorb and lock in liquids (such as menstrual blood) and keep the surface dry.
Technique Date
|
Item |
Unit |
Specification |
Testing Results |
|
Appearance |
|
White Granules |
White Granules |
|
Free Absorbency |
g/g |
≥51 |
55 |
|
Retention Capacity |
g/g |
≥31 |
34 |
|
Residual Monomer |
PPM |
≤500 |
490 |
|
Moisture Conten |
wt% |
≤4.5 |
4.1 |
|
Absorption under pressure |
0.3psi ml/g |
≥27 |
29 |
|
0.7psi ml/g |
≥18 |
20 | |
|
Absorption Rate(Voltex) |
S |
≤55 |
38 |
|
Bulk density |
g/ml |
0.5-0.7 |
0.6 |
|
PH Value |
|
5.5-6.5 |
6 |
|
Particle Size Distribution |
Wt % |
|
|
|
850μm on % |
≤2 |
0 |
|
|
150μm through % |
0 |
4.3 |
What is SAP in sanitary pads?
SAP Application
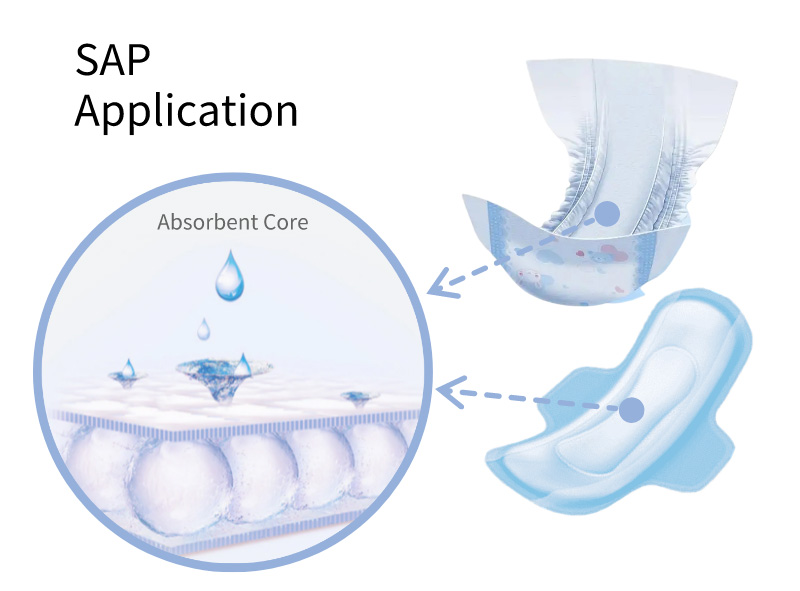
Widely used in the absorption core of
Baby Diapers
Adult Diapers
Sanitary Napkins
Nursing Pads
Other Hygiene Products
Tips: How SAP works in sanitary napkins
SAP is typically used as the raw materials of sanitary napkin. It helps improve capacity for better retention in a sanitary napkin, allowing the sanitary napkin to be thinner with improved performance and less usage of pine fluff pulp. The molecular structure of the polyacrylate has sodium carboxylate groups hanging off the main chain. When it comes in contact with water, the sodium detaches itself, leaving only carboxyl ions. Being negatively charged, these ions repel one another so that the polymer unwinds and absorbs water, which is attracted by the sodium atoms. The polymer also has cross-links, which effectively leads to a three-dimensional structure. It has high molecular weight of more than a million; thus, instead of getting dissolved, it solidifies into a gel. The Hydrogen in the water (H-O-H) is trapped by the a crylate due to the atomic bonds associated with the polarity forces between the atoms. Electrolytes in the liquid, such as salt minerals (urine contains 0.9% of minerals), reduce polarity, thereby affecting super absorbent properties, especially with regard to the super absorbent capacity for liquid retention.Linear molecular configurations have less total capacity than non-linear molecules but, on the other hand, retention of liquid in a linear molecule is higher than in a non-linear molecule, due to improved polarity.
Examples of a linear molecule


Packaging& Delivery

Test Vedio
Quanzhou Niso Industry Co.,Ltd is your best choice for diaper raw material, sanitary napkin material and disposable hygiene products in China.